- Credits
- Section Writers: Dr. Om J Lakhani, Dr. Amitabh Sur
- Section Editor: Dr. Om J Lakhani
Support us: the new "Notes in Endocrinology" App
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
- Support you by Becoming a YouTube member (Click here)
-
Q. Which electrolyte must be monitored explicitly for patients on PPI?
- Magnesium
- Hypomagnesemia is common with the use of PPI
- The levels of the same must be done for patients to be given PPI for >1 year
- For patients on drugs that can potentially cause Hypomagnesemia like diuretics, the levels must be regularly monitored
- You must especially be careful of patients with long QT syndrome
-
Q. What about the correlation with Calcium?
- PPI mainly impact the absorption of Calcium carbonate
- Hence if you are planning to supplement calcium, it is better to give Calcium citrate instead of carbonate #Clinicalpearl
-
Q. Which vitamin deficiency is common with the use of PPI?
- Vitamin B12 deficiency
- However, supplements are not impacted by PPI
-
Q. What about iron deficiency?
- Non-heme iron absorption is affected by the use of PPI
- However, it generally does not lead to clinically significant anemia
- If the patient is on iron supplements, the dose requirement may increase
-
Q. What is the dreaded renal complication of PPI?
- PPI can cause Acute interstitial nephritis
- it is not dose-dependent, and recurrence and exacerbation can occur with re-exposure
-
Q. Do PPI cause chronic kidney disease?
- There is a concern that long term PPI use can lead to CKD
- However, this is controversial, and the exact mechanism for the same has not been determined [1]
- "In 93,335 patients in the AKI cohort, 16,593 of whom were exposed to PPIs, the incidence rate of AKI was higher in the PPI group than nonusers (36.4 vs. 3.54 per 1000 person-years, p<0.0001, respectively).
- In adjusted models, PPI exposure was associated with an increased risk of AKI (adjusted odds ratio [aOR] 4.35, 95% confidence interval [CI] 3.14-6.04, p<0.0001).
- In 84,600 patients in the CKD cohort, 14,514 of whom were exposed to PPIs, the incidence rate of CKD was higher in the PPI group than nonusers (34.3 vs 8.75 per 1000 person-years, p<0.0001, respectively).
- In adjusted models, PPIs were associated with a higher risk of CKD compared with controls (aOR 1.20, 95% CI 1.12-1.28, p<0.0001).
- Associations between PPI use and AKI and CKD persisted in propensity score-matched analyses.
- The use of PPIs is associated with an increased risk of incident AKI and CKD. This relationship could have a considerable public health impact; therefore, health care provider education and deprescribing initiatives will be necessary to raise awareness and reduce the health care burden.
-
Q. What is the dreaded skin involvement with PPI?
- PPI can lead to Drug-induced lupus
- It is self-limiting in a few weeks after discontinuation of the medication
- It is subacute in onset
-
Q. Does PPI lead to an increased risk of COVID-19 infection?
- This has been reported in some studies
- However, confounding factors cannot be ruled out completely
- [2]
- Of 53,130 participants, 3,386 (6.4%) reported a positive COVID-19 test. In regression analysis, individuals using PPIs up to once daily (aOR 2.15; 95% CI, 1.90–2.44) or twice daily (aOR 3.67; 95% CI, 2.93–4.60) had significantly increased odds for reporting a positive COVID-19 test when compared with those not taking PPIs.
- Individuals taking histamine-2 receptor antagonists were not at elevated risk.
- We found evidence of an independent, dose-response relationship between the use of antisecretory medications and COVID-19 positivity;
- individuals taking PPIs twice daily have higher odds for reporting a positive test when compared with those using lower-dose PPIs up to once daily, and those taking the less potent histamine-2 receptor antagonists are not at increased risk.
- These findings emphasize good clinical practice that PPIs should only be used when indicated at the lowest effective dose, such as the approved once-daily label dosage of over-the-counter and prescription PPIs.
- Further studies examining the association between PPIs and COVID-19 are needed.
-
Q. What is the impact of PPI in patients with hypothyroidism on Levothyroxine supplementation?
- A study conducted by Issac Sachmechi et al. concluded that the introduction of PPI in patients with hypothyroidism on LT4 lead to a statistically significant increase in the TSH levels after 2 months [3]
- Source: Medscape
- "Patients with hypothyroidism receiving levothyroxine who are euthyroid may need subsequent thyroid function tests following the initiation of a PPI, especially if symptoms of hypothyroidism emerge.
- Although the precise effect of PPIs on levothyroxine pharmacokinetics has not been fully elucidated, patients may require an increase in their levothyroxine dose several months into PPI therapy. This interaction is not entirely predictable, but it appears more likely after six months of PPI therapy. Future studies should control for the many confounding variables known to influence levothyroxine absorption."
-
Q. Does the prolonged use of PPI lead to Gastric neuroendocrine tumors?
- Though PPI use can lead to an increase of gastrin levels, increased risk of NET has not be observed with short term use
- However, increased vigilance is required for patients taking PPI for more than ten years and those who develop chronic gastritis secondary to the same.
- Increased vigilance is also required for those having H. Pylori infection
-
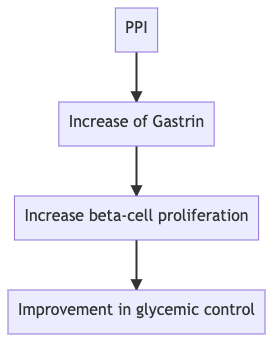
Q. Does the use of PPI have any impact on glycemic control in patients living with diabetes?
- Yes
- The use of PPI in patients with diabetes has been found to improve glycemic control, according to a recent meta-analysis
- Carol Chiung-Hui Peng et al :
- "Compared with standard therapy, add-on PPI was associated with a significant decrease in HbA1c (WMD, −0.36 %; 95% CI, −0.68 to −0.05; P = 0.025) and FBG (WMD, −10.0 mg/dL; 95% CI, −19.4 to −0.6; P = 0.037). PPI use did not reduce the risk of incident diabetes (pooled RR, 1.10; 95% CI, 0.89 to 1.34; P = 0.385)" [4]
-
Q. What could be the possible mechanism by which the use of PPI could have a beneficial effect on glycemic control?
- This is likely because of the impact of gastrin
-
See below
-
-
**PPI and Osteoporosis **
-
Q. Is the prolonged use of PPI associated with an increased risk of Osteoporosis?
-
Yes
-
A meta-analysis published in the BMJ shows about 30% increased risk of fracture with the use of PPI [5]
-

-
The risk is significantly more in patients with CKD
-
Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved
- The current review suggests that the relationship between long-term PPI use and fracture is still unclear; however, the risk is more apparent in patients with strong secondary risk factors of osteoporosis, such as renal dysfunction [6]
-
-
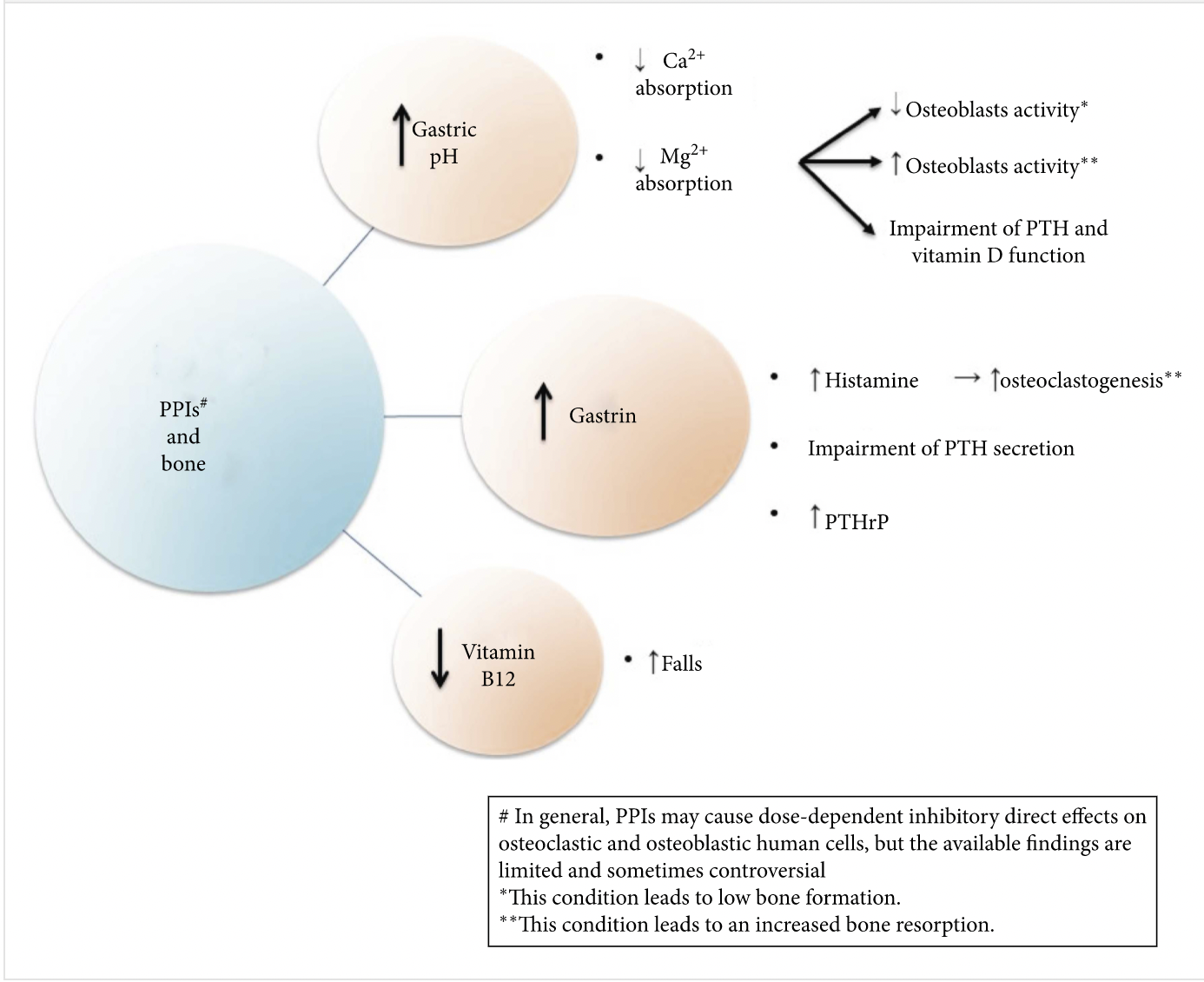
Q. What is the possible mechanism behind PPI causing fracture?
Video
Hart E, Dunn TE, Feuerstein S, Jacobs DM. Proton Pump Inhibitors and Risk of Acute and Chronic Kidney Disease: A Retrospective Cohort Study. Pharmacotherapy. 2019 Apr;39(4):443-453. DOI: 10.1002/phar.2235. Epub 2019 Mar 21. PMID: 30779194; PMCID: PMC6453745. ↩︎
Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol. 2020 Oct;115(10):1707-1715. DOI: 10.14309/ajg.0000000000000798. PMID: 32852340; PMCID: PMC7473791 ↩︎
Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007 Jul-Aug;13(4):345-9. DOI: 10.4158/EP.13.4.345. PMID: 17669709 ↩︎
Carol Chiung-Hui Peng, Yu-Kang Tu, Gin Yi Lee, Rachel Huai-En Chang, Yuting Huang, Khulood Bukhari, Yao-Chou Tsai, Yunting Fu, Huei-Kai Huang, Kashif M Munir, Effects of Proton Pump Inhibitors on Glycemic Control and Incident Diabetes: A Systematic Review and Meta-Analysis, The Journal of Clinical Endocrinology & Metabolism, 2021;, dgab353, https://doi.org/10.1210/clinem/dgab353 ↩︎
Khalili H, Huang ES, Jacobson BC, Camargo CA, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012 Jan 31;344. ↩︎
Thong BKS, Ima-Nirwana S, Chin KY. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. Int J Environ Res Public Health. 2019 May 5;16(9):1571. DOI: 10.3390/ijerph16091571. PMID: 31060319; PMCID: PMC6540255 ↩︎
Thong BK, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. International journal of environmental research and public health. 2019 Jan;16(9):1571. ↩︎
Briganti SI, Naciu AM, Tabacco G, Cesareo R, Napoli N, Trimboli P, Castellana M, Manfrini S, Palermo A. Proton Pump Inhibitors and Fractures in Adults: A Critical Appraisal and Review of the Literature. International Journal of Endocrinology. 2021 Jan 15;2021 ↩︎