- Credits
- Section Writer: Dr. Om J Lakhani
- Section Editor: Dr. Om J Lakhani
Support us:
- Support you by Becoming a YouTube member (Click here).
- Premium Membership- Download PDF version of Notes, Get ad free video and more
- Consultant Membership- Above plus Download Powerpoint presentation of the notes and get access to EndoAI for Free
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
-
Q. What are the current FDA recommendations with the use of SGLT2i in the perioperative period?
- FDA recommends the following:
- Stop Canagliflozin, Dapagliflozin, Empagliflozin and Bexagliflozin at least 3 days before surgery
- Stop Ertugliflozin at least 4 days before surgery
- FDA recommends the following:
-
Q. What is the basis for the above recommendation in terms of timing?
- The typical half-life of SGLT2i is 11-13 hours
- So they are looking at >5 half-lives
-
Q. What is the main concern with the use of SGLT2i in the perioperative period?
- Risk of Euglycemic Ketoacidosis (eDKA)
-
Q. What is SAPKA ?
- SGLT2 Inhibitor Associated Perioperative Ketoacidosis (SAPKA)
-
Q. When does Euglycemic Ketoacidosis (eDKA) generally occur in these cases?
- They generally occur in the postoperative period
-
Q. True or false, the number of cases reported with Euglycemic Ketoacidosis (eDKA) in literature are increasing over a period of time?
- True
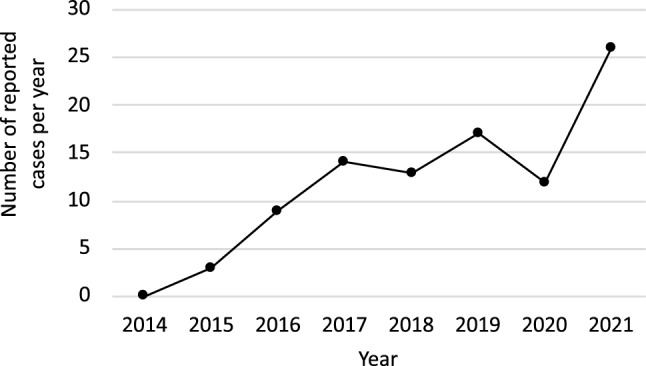
-
Q. What is the definition of Euglycemic Ketoacidosis (eDKA) ?
- RBS <252 mg/dl with
- pH <7.3
- Bicarbonate <15 meq/l
- Anion gap >12
- RBS <252 mg/dl with
-
Q. What are the typical Beta-Hydroxybutyrate levels in patients with Euglycemic Ketoacidosis (eDKA) ?
- Most cases of Euglycemic Ketoacidosis (eDKA) have reported Beta-Hydroxybutyrate levels to be more than 2 mmol/l
- This is more than 20 mg/dl
-
Q. Why do SGLT2i predispose to Diabetic Ketoacidosis ?
- See the diagram below
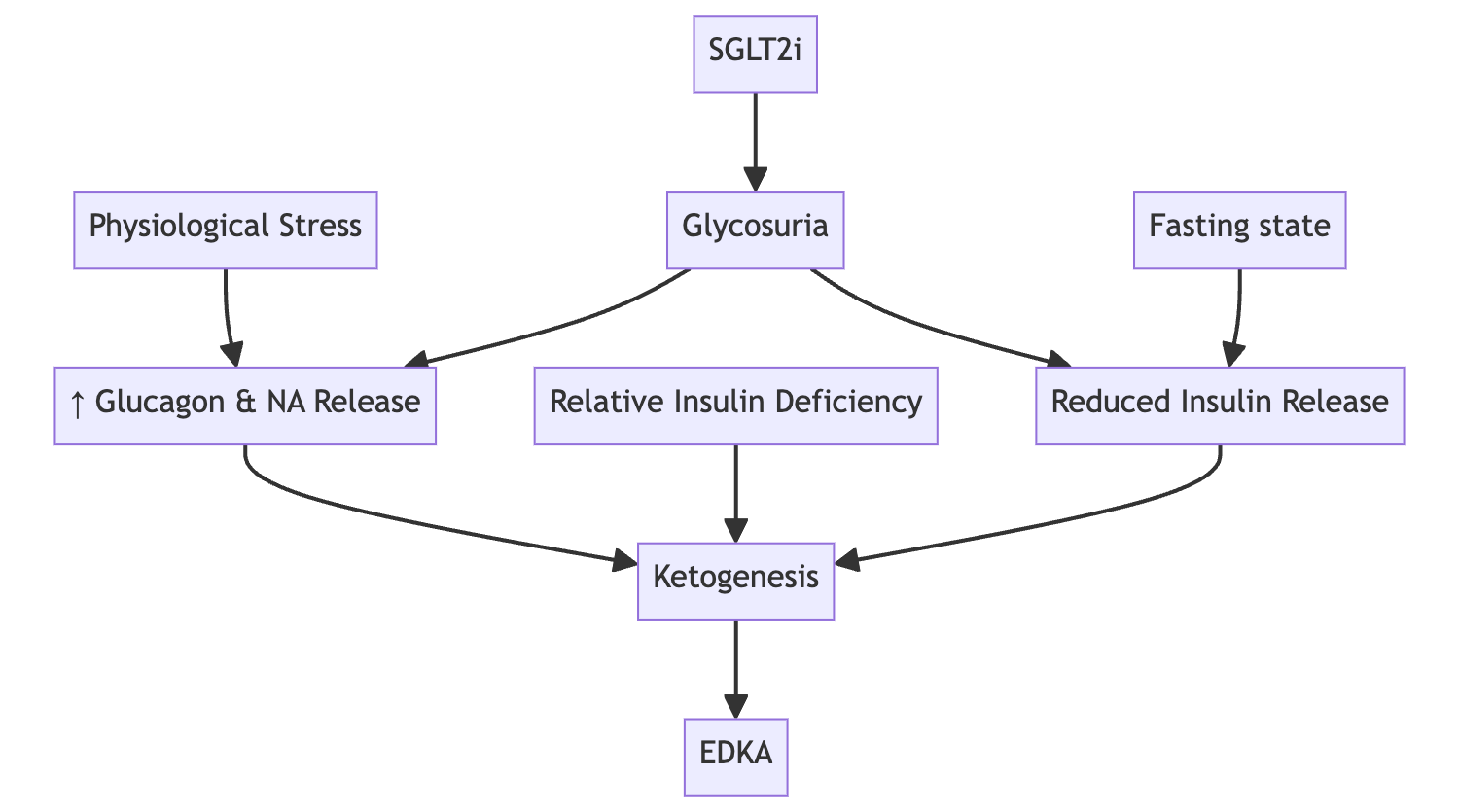
-
Q. Which is the other mechanism recently being recognized as an important cause of increased ketones in patients on SGLT2i ?
- Reabsorption of ketone bodies from urine is seen with the use of SGLT2i
- This also seems to be an important process in this mechanism
-
Q. Do SGLT2i impact Glucagon levels?
- Yes
- Patients on SGLT2i have higher glucagon levels
- However, this impact is indirect
-
Q. What are the symptoms of Euglycemic Ketoacidosis (eDKA) ?
- Symptoms may be more subtle sometimes
- Respiratory distress
- Gastrointestinal disturbances
- Malaise
- Symptoms may be more subtle sometimes
-
Q. What are the key points we have learned about SAPKA from the meta-analysis by Seki et al?
- Type of Surgery:
- Bariatric surgery was the most common type of surgery leading to the diagnosis
- Time to Diagnosis:
- Most patients (25.3%) were diagnosed on the first postoperative day (POD 1).
- Trigger for Identification:
- Laboratory data was the most frequent trigger for identification (29 cases).
- Nausea and/or vomiting were reported in 21 cases.
- Breath shortness/dyspnea was a trigger for 20 cases.
- Fatigue or malaise was reported by 14 patients.
- Tachycardia and tachypnea were observed in 16 and 15 cases respectively.
- Blood Ketones and Median BGA Data:
- The median blood BHB level was 5.8 mmol/L
- The mean anion gap was 23 meq/l
- The median pH value at the time of diagnosis was 7.16, with a range from 6.82 to 7.29.
- Type of Surgery:
-
Q. Do patients receiving SGLT2i for non-diabetes indications like heart failure and CKD are also at risk of Euglycemic Ketoacidosis (eDKA) ?
- No
- Non-diabetic patients have NOT been reported to have Euglycemic Ketoacidosis (eDKA)
- Update- Just one case has been reported as suggested by the meta-analysis by Seki et al (see reference below)
- Hence in such cases it may be okay to continue SGLT2i preoperatively
- However they may develop stress hyperglycemia in the perioperative period and hence this has to be kept in mind
- The guideline recommends getting an anion gap done post-operatively in such patients to rule out Euglycemic Ketoacidosis (eDKA)
-
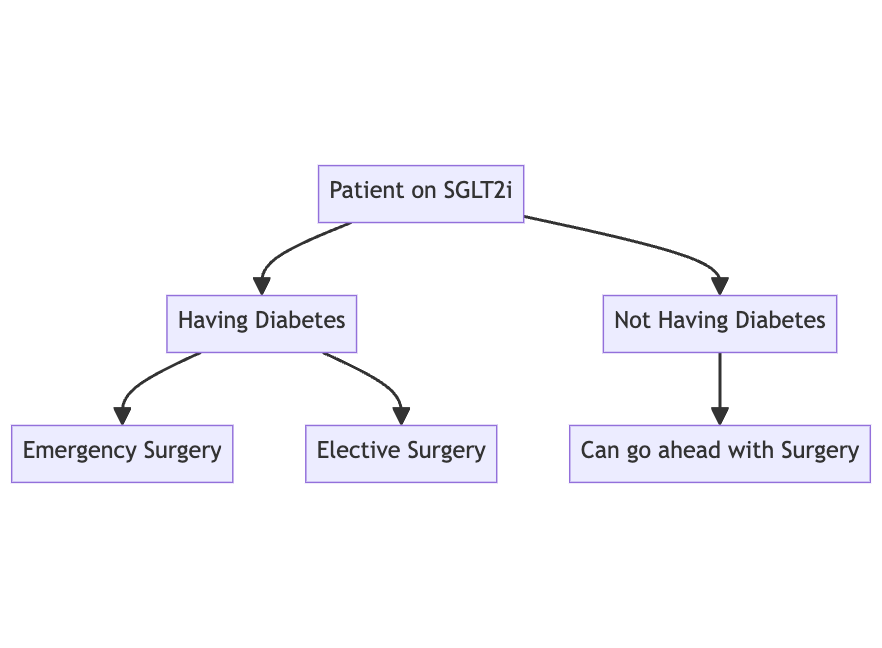
Q. What is the perioperative recommendation for patients with diabetes in whom the SGLT2i have been stopped at an adequate period prior to surgery?
- The patient can go ahead with surgery with perioperative use of insulin as appropriate
- However if post-operatively the diet is not resumed within 2 hours it is recommended to get an anion gap done
- It has to be repeated every 12 hourly after till the carbohydrate in diet is resumed
-
Q. Why is this required even for patients in whom the drug has been adequately stopped?
- This is because the effect of SGLT2i have been shown to persist for a longer time even in patients in whom the drug was discontinued and even with normal renal function
- Cases of Euglycemic Ketoacidosis (eDKA) have been reported as long as 10 days after discontinuation of the drug
-
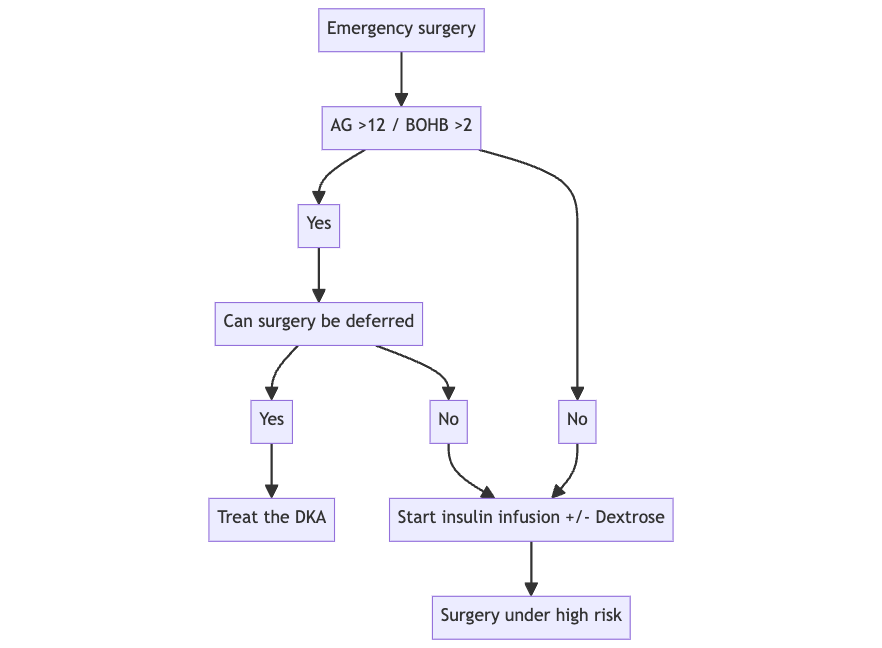
Q. What to do in diabetic patients on SGLT2i when emergency surgery is required?
- Check Beta-Hydroxybutyrate and anion gap to ensure that the patient is not currently in DKA
- If the patient is having DKA- see if emergency surgery can be postponed
- Else it is recommended to carry out the procedure as usual looking at risk vs benefit
-
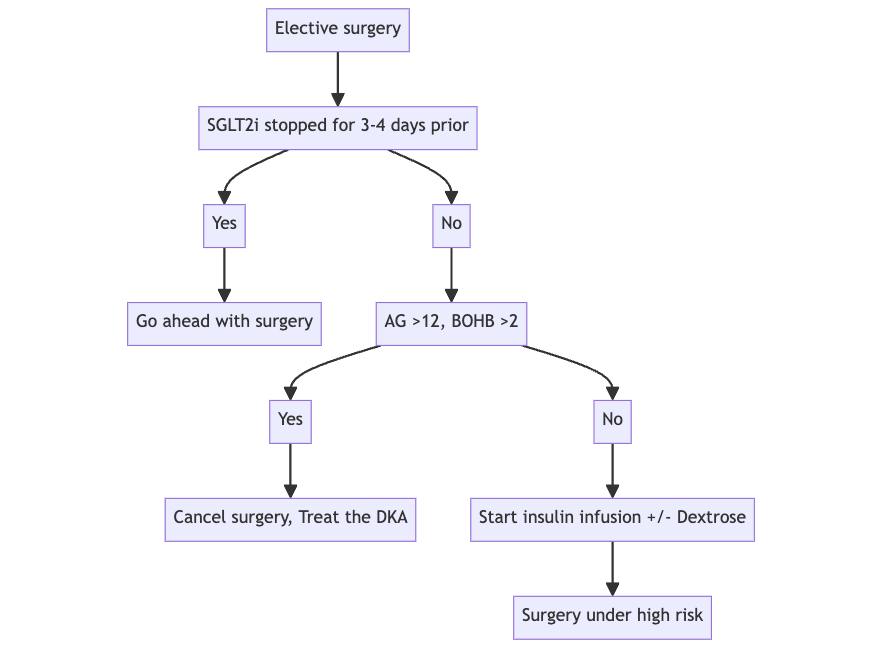
Q. What is done if the patient shows up for surgery without stopping the medication as suggested?
- Check anion gap
- If >12 → postpone surgery
- If <12 → can go ahead with surgery with good insulinization but make sure that the patient is not having a fasting period of >12 hours
- Check anion gap
-
Q. Give an outline of what to do perioperatively in patients with SGLT2i undergoing surgery?
- Step 1:
- Step 2A - Emergency surgery
- Step 2B- Elective surgery
- Step 1:
-
Q. What should be done intraoperatively in all these patients?
- Monitor blood glucose and give insulin infusion
- With dextrose if glucose <200 mg/dl
- Monitor blood glucose and give insulin infusion
-
Q. What is done post-operatively?
- It would be a good idea to check the anion gap - if there is going to be a delay of >2 hrs on starting carbohydrate meal
- If the patient is to be discharged immediately- then it is a good idea to check the glucose and anion gap at discharge- consider insulin if required
- After day of the surgery if the patient is taking orally- they can restart the SGLT2i
- If the patient is going to be admitted- then continue insulin infusion with dextrose till oral intake is resumed and then put the patient on subcutaneous insulin as per protocol
- Check the anion gap 4-12 hourly depending on the perioperative risk
-
Q. Which surgery are considered high risk vs low risk in terms of risk of Euglycemic Ketoacidosis (eDKA) ?
- Factors suggesting low risk:
- Procedure <1 hr for GA, local or regional anesthesia
- Total anticipated NPO duration <12 h
- Pre-op A1C <8%
- Pre-op blood glucose <150 mg/dl
- Not on insulin as outpatient
- No significant background comorbidity
- Factors suggesting higher risk:
- Procedure >1 hour or requiring general anesthesia
- Total anticipated NPO duration >12 h
- Pre-op A1C >8
- Pre-op blood glucose >150 mg/dl
- On insulin as outpatient
- Significant background comorbidity (e.g., trauma, MI, etc).
- Factors suggesting low risk:
-
Q. How do you manage Euglycemic Ketoacidosis (eDKA) ?
- Management is the same as other causes of DKA, only that you might have to give dextrose containing IV fluids since the glucose <250 mg/dl
- Here is the outline of stepwise management:
- Step 1: Stop inciting agent, if applicable (e.g., SGLT2i)
- Step 2: Start fluid replacement with monitoring of electrolytes and ketones
- Step 3: Start continuous insulin infusion
- Step 4: Start dextrose administration
-
Q. Apart from insulin and dextrose, which drug has been proposed for potential treatment for Euglycemic Ketoacidosis (eDKA) ?
- Somatostatin
- Somatostatin was suggested for treatment of Diabetic Ketoacidosis way back in 1980, but hasn't been used much because of better insulin available
- With the advent of Euglycemic Ketoacidosis (eDKA) , the use of the same is emerging as evidenced by the case report Torre et al
- The mechanism of action proposed is reduction of glucagon levels by the use of this agent
-
Q. Is there any role of somatostatin analogue octreotide in Euglycemic Ketoacidosis (eDKA) ?
- There are mixed data to support the use of Octreotide for this purpose
- Several papers (Burge et al, Yun et al) have shown little or no benefit of the use of Octreotide in conventional Diabetic Ketoacidosis
- One paper by Diem et al shows that it is useful to prevent recurrence of DKA in patients with recent DKA
- References:
-
- Raiten JM, Morlok A, D'Ambrosia S, Ruggero MA, Flood J. Perioperative Management of Patients Receiving Sodium Glucose Co-Transporter-2 Inhibitors: Development of a Clinical Guideline at a Large Academic Medical Center. Journal of Cardiothoracic and Vascular Anesthesia. 2023 Oct 10.
-
- Torre A, Bisogno N, Botta C, Caiazza A, D’Angelo F, Del Giudice L, Fiorentini P, Marzano L, Nigro R, Sassone D, Torre P. Treatment of a Severe Form of Euglycemic Ketoacidosis in a Patient Treated with SGLT-2 Inhibitors with the Aid of Somatostatin.
-
- Burge MR, Qualls CR, Kramer K, Colleran K, Schade DS. Utility of Subcutaneous Octreotide in the Early Recovery from Diabetic Ketoacidosis in Acutely Ill Type 1 Diabetes Patients. J Diabetes Metab Disord Control. 2016;3(5):00079.
-
- Yun YS, Lee HC, Park CS, Chang KH, Cho CH, Song YD, Lim SK, Kim KR, Huh KB. Effects of Long-Acting Somatostatin Analogue (Sandostatin) on Manifest Diabetic Ketoacidosis. Journal of Diabetes and its Complications. 1999 Sep 1;13(5-6):288-92.
-
- Diem P, Robertson RP. Preventive Effects of Octreotide (SMS 201-995) on Diabetic Ketogenesis during Insulin Withdrawal. Br J Clin Pharmacol. 1991 Nov;32(5):563-7. doi: 10.1111/j.1365-2125.1991.tb03952.x. PMID: 1954071; PMCID: PMC1368631.
-
- Ng KE. Management of Euglycemic Diabetic Ketoacidosis.
-
- Seki H, Ideno S, Shiga T, Watanabe H, Ono M, Motoyasu A, Noguchi H, Kondo K, Yoshikawa T, Hoshijima H, Hyuga S. Sodium-Glucose Cotransporter 2 Inhibitor-Associated Perioperative Ketoacidosis: A Systematic Review of Case Reports. Journal of Anesthesia. 2023 Feb 27:1-9.
-