- Credits
- Section Writer: Dr. Om J Lakhani
- Section Editor: Dr. Om J Lakhani
Support us:
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
- Support you by Becoming a YouTube member (Click here)
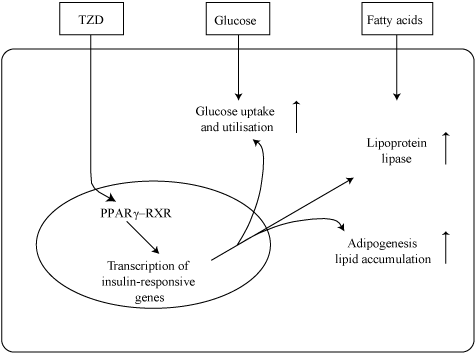
- Q. How do Thiazolidinediones act ?
- They activate PPAR-gamma
- This leads to the transcription of insulin-responsive genes that
- Increase adipogenesis
- Activate Lipoprotein lipase
- Increase peripheral glucose uptake and utilization
- All this leads to reduced peripheral insulin resistance
- Q. What is the structure of Lobeglitazone?
- It is modified from Rosiglitazone
- The structure also includes a p-methoxyphenyl group at the 4-position of the pyrimidine moiety, which enhances its binding affinity for PPAR γ.
- Q. How is the structure different from Rosiglitazone?
- Lobeglitazone has a p-methoxyphenyl group at the 4-position of the pyrimidine moiety, which is not present in Rosiglitazone
- This modification contributes to the enhanced binding affinity of lobeglitazone for PPAR γ, with docking analysis suggesting that the binding affinity of lobeglitazone is 12 times higher than that of Rosiglitazone and Pioglitazone.
- Q. What is the impact of Thiazolidinediones on triglyceride and Free Fatty acid?
- They reduce both triglyceride and Free Fatty acid
- Q. What are the beneficial effects of Lobeglitazone on adipose tissue?
- Lobeglitazone induces differentiation of preadipocytes into insulin-sensitive small adipocytes.
- It redistributes from visceral to subcutaneous adipose depots.
- It induces the differentiation of white adipocytes to beige adipocytes and is associated with the development of brown adipocytes.
- Q. What are the other pleiotropic benefits of Lobeglitazone?
- Preclinical studies suggest that lobeglitazone might have beneficial effects on various organs, including the heart, liver, kidney, and lungs.
- Lobeglitazone improved cardiac hypertrophy and dysfunction in diabetic rats. It also reduced Atherosclerosis risk in mice
- It improved hepatic steatosis and inflammation in mice.
- It reduced airway hyperresponsiveness in a murine model of asthma.
- It improved renal fibrosis in mice by inhibiting transforming growth factor-β/Smad signaling.
- Q. What is the effect of Lobeglitazone on the risk of osteoporosis?
- Studies with lobeglitazone found that it did not inhibit osteoblast differentiation in vitro and had no adverse effect on bone mineral density (BMD) in mice. Therefore, it is suggested that lobeglitazone has no adverse effect on bone health and does not increase the risk of osteoporosis.
- Q. What about the risk of bladder cancer with Lobeglitazone?
- The risk of bladder cancer with Lobeglitazone is considered low based on preclinical studies and its negligible urinary excretion.
- Clinical trials conducted to date have also shown favorable results on the safety of Lobeglitazone.
- However, further research is needed to assess cardiovascular outcomes and the risk of bladder cancer with Lobeglitazone as the small sample sizes and short study durations cannot definitively confirm the long-term clinical benefits and risks.
- Q. How is Lobeglitazone metabolized in the human body?
- Lobeglitazone is mainly metabolized in the liver through cytochrome P450 (CYP) isoforms, including CYP3A4 , 2C19, and 2D6.
- Q. What is the half-life of the drug?
- About 7-9 hours
- Q. Tell me about the interaction between Lobeglitazone with food intake.
- A slight increase in the AUC for lobeglitazone was observed in subjects receiving a high lipid diet compared with fasting subjects.
- The slight increase in bioavailability with a high lipid diet was not considered to be clinically significant.
- In a study evaluating a fixed-dose combination tablet containing lobeglitazone 0.5 mg plus metformin extended-release 1,000 mg, lobeglitazone Cmax decreased by approximately 32% when administered after a high-fat meal compared with the fasting state.
- However, there was no significant difference between the fed and fasted states concerning lobeglitazone AUC up to the last sampling time.
- So basically the interaction of food is negligible
- Q. Tell me about the use of Lobeglitazone in patients with CKD, especially ESRD.
- Lobeglitazone can be used in patients with renal insufficiency without dose reduction, as it is mainly metabolized by the liver with negligible renal excretion.
- An open-label, parallel-group, single-dose, non-randomized study was conducted to evaluate the pharmacokinetic profile of lobeglitazone in patients with renal impairment, including end-stage renal disease (ESRD).
- The geometric means for Cmax and AUC in patients with ESRD were similar to those for subjects with normal renal function.
- Cmax and AUC inf values for M7 (the active metabolite of lobeglitazone) were 1.22 and 1.31 times higher in the ESRD group compared with the control group, but overall, the pharmacokinetic profiles did not differ significantly between the two groups.
- Q. Tell me about any significant drug interactions with Lobeglitazone.
- According to the mentioned studies, there are significant drug interactions of Lobeglitazone with Warfarin and Ketoconazole. The pharmacokinetics and pharmacodynamics of Warfarin are affected by Lobeglitazone, and Lobeglitazone increases exposure to Warfarin. On the other hand, Ketoconazole affects the pharmacokinetics of Lobeglitazone, and co-administration of these drugs increases the plasma concentration of Lobeglitazone. Therefore, caution should be exercised if Lobeglitazone is prescribed along with these drugs. No other significant drug interactions of Lobeglitazone have been reported in the studies mentioned.
- Q. What is the dose and brand of Lobeglitazone available in India?
- Lobg - 0.5 mg
- Q. What is the dose of Lobeglitazone?
- Dose is 0.5 mg once a day
- Q. How much of an HbA1c reduction do you expect with Lobeglitazone?
- Lobeglitazone at a dose of 0.5 mg/day has been shown to reduce HbA1c by 0.44% after 24 weeks of monotherapy, compared to a placebo group.
- In combination therapy with metformin, lobeglitazone has been shown to reduce HbA1c by 0.74% after 24 weeks. There is also evidence suggesting that lobeglitazone has similar glycemic efficacy to pioglitazone.
- Q. How does Lobeglitazone compare to Pioglitazone in terms of potency and efficacy in glucose-lowering effect?
- Some studies suggest that pioglitazone may have a more potent glucose-lowering effect, but these studies recruited patients with higher baseline HbA1c.
- A direct comparison between lobeglitazone and pioglitazone in terms of glucose-lowering efficacy has not been conducted.
- An indirect comparison based on previous prospective randomized, controlled studies showed that both lobeglitazone and pioglitazone had similar reductions in HbA1c levels when used as monotherapy or add-on therapy to metformin.
- Q. Tell me more about the clinical impact of Lobeglitazone on NAFLD.
- Lobeglitazone is a peroxisome proliferator-activated receptor γ (PPARγ) agonist that has shown potential in treating non-alcoholic fatty liver disease (NAFLD) in preclinical studies.
- In a study on diet-induced obese mice, lobeglitazone treatment attenuated hepatic steatosis, reduced serum levels of liver enzymes, and improved glucose intolerance.
- Lobeglitazone was investigated in a single-arm study for its effects on non-alcoholic fatty liver disease (NAFLD).
- The study included 50 patients with type 2 diabetes mellitus (T2DM) and NAFLD, defined as controlled attenuation parameter (CAP) ≥250 dB/m.
- Patients received lobeglitazone 0.5 mg once daily for 24 weeks.
- After 24 weeks, mean CAP significantly decreased (from 313.4±30.9 to 297.8±39.1 dB/m, P=0.016).
- Liver enzymes including aspartate transaminase, alanine transaminase, and gamma-glutamyl transferase also decreased.
- The therapeutic effect of lobeglitazone on NAFLD was independent of its glycemic efficacy.
- Q. What is the controlled attenuation parameter with regards to Fibroscan?
- Controlled attenuation parameter (CAP) is a novel physical parameter based on the properties of ultrasonic signals acquired by the Fibroscan®.
- It uses the postulate that fat affects ultrasound propagation and is a measure of ultrasound attenuation at the central frequency of the Fibroscan® M or regular probe.
- It is a promising tool for the noninvasive detection of hepatic steatosis
- Q. What is LSM with regards to Fibroscan?
- LSM stands for Liver Stiffness Measurement (LSM). It is a parameter obtained using FibroScan for the diagnosis and quantification of liver fibrosis by measuring mechanical or ultrasound shear wave propagation through the hepatic parenchyma
- #Clinicalpearl- with regards to Fibroscan
- Controlled attenuation parameter (CAP) is a measure of Liver fat
- LSM is a measure of liver fibrosis
- Q. Tell me about the risk of pedal edema with Lobeglitazone.
- Peripheral edema is a known side effect associated with thiazolidinediones (TZDs), including lobeglitazone.
- In clinical studies using lobeglitazone monotherapy for 24 weeks, 3.6% of patients experienced peripheral edema.
- The incidence of peripheral edema was 3.1% of patients by the end of the monotherapy extension study (total 52 weeks).
- When lobeglitazone was administered in combination with metformin, 3.9% of patients experienced edema within 24 weeks, with no cases of severe edema.
- The incidence of peripheral edema with lobeglitazone seems to be acceptable compared to clinical studies using pioglitazone monotherapy, which ranged from 3.6% to 28.9%.
- Q. How much weight gain do you expect with Lobeglitazone?
- About 0.9-1 kg for 24 weeks
- Q. Give the key conclusions with regards to Lobeglitazone.
- Preclinical studies suggest that lobeglitazone may have beneficial effects on various organs, including the liver, lungs, and kidneys. Its role in NASH/NAFLD is particularly interesting
- Clinical studies are needed to evaluate the potential role of lobeglitazone in these settings.
- Lobeglitazone did not inhibit osteoblast differentiation in vitro and had no adverse effect on bone mineral density (BMD) in mice, contrary to rosiglitazone which reduced BMD.
- Long-term studies in rats and mice suggested that lobeglitazone had a low carcinogenic potential and did not induce urothelial tumors.
- Lobeglitazone clearance was mainly mediated by liver metabolism and the ratio of renal excretion was predicted to be <1.0%. It can also be used in patients with ESRD
- The main cytochrome P450 (CYP) isoforms involved in the hepatic metabolism of lobeglitazone were CYP3A4 hence drug interactions can be expected
- It is given in a dose of 0.5 mg once a day