- Credits
- Section Writer: Dr. Om J Lakhani
- Section Editor: Dr. Om J Lakhani
Support us:
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
- Support you by Becoming a YouTube member (Click here)
- Q. What kind of dual action does Imeglimin have?
- Imeglimin's dual action involves:
- Amplification of glucose-stimulated insulin secretion (GSIS) and preservation of β-cell mass
- Enhanced insulin action, including inhibition of hepatic glucose output and improvement in insulin signaling in liver and skeletal muscle
- Insulin-sensitizing effect in liver and skeletal muscle, evidenced by increased PKB (Akt) phosphorylation in response to exogenous insulin, reduced hepatic steatosis, and increased in vivo C-2-deoxy glucose uptake into skeletal muscles of STZ-diabetic rats.
- It improves beta-cell function as well as reduces insulin resistance
- Imeglimin's dual action involves:
- Q. What is the role of mitochondrial dysfunction in the pathogenesis of diabetes?
- Mitochondrial dysfunction is a key component of T2D pathophysiology
- It is associated with impaired insulin secretion, insulin resistance, and increased oxidative stress
- Mitochondrial dysfunction can lead to decreased ATP production, increased ROS production, and impaired glucose metabolism
- Imeglimin is a novel oral agent designed to target key components of T2D pathophysiology
- Imeglimin has been shown to modulate mitochondrial function, reduce ROS, and protect cells from death in response to insults that include cytokines or hyperglycemia
- Imeglimin may have the potential to address important complications of diabetes, including cardiac dysfunction and nephropathy.
- Q. What is the impact of the drug on beta-cell dysfunction?
-
- It enhances Glucose stimulated insulin release
-
- Also prevents the loss of beta-cell mass
-
- Q. What is Stumvoll Index ?
- It is a measure of insulin sensitivity like QUICKI
- Q. How does Imeglimin produce its clinical effect?
- Imeglimin targets defective cellular energy metabolism, a root cause of type 2 diabetes
- Its mechanism involves correcting mitochondrial dysfunction, a common element of type 2 diabetes pathogenesis
- Imeglimin rebalances respiratory chain activity, reducing reactive oxygen species formation and preventing mitochondrial permeability transition pore opening
- In islets from diseased rodents with type 2 diabetes, Imeglimin enhances glucose-stimulated ATP generation and induces NAD synthesis via the 'salvage pathway'
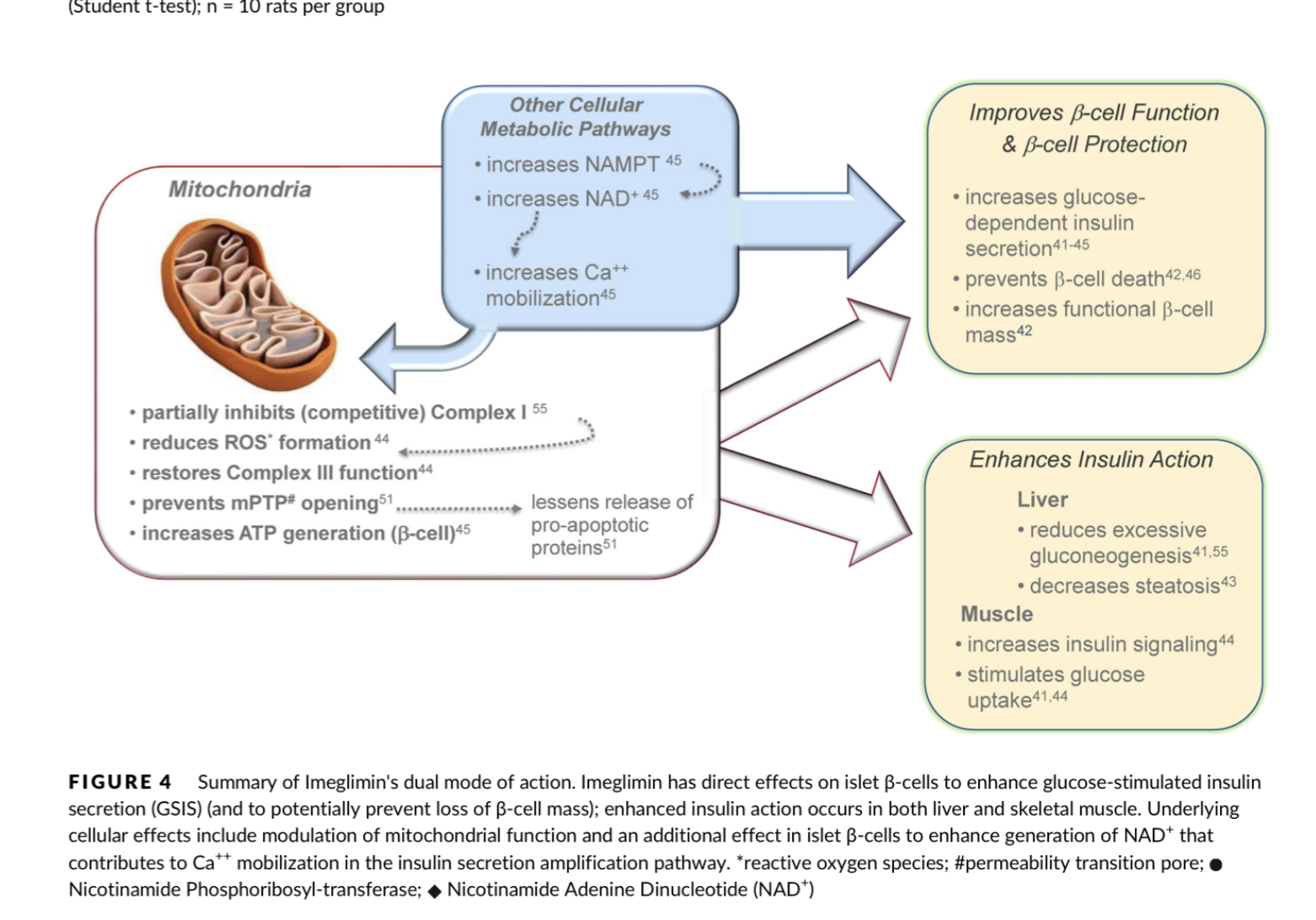
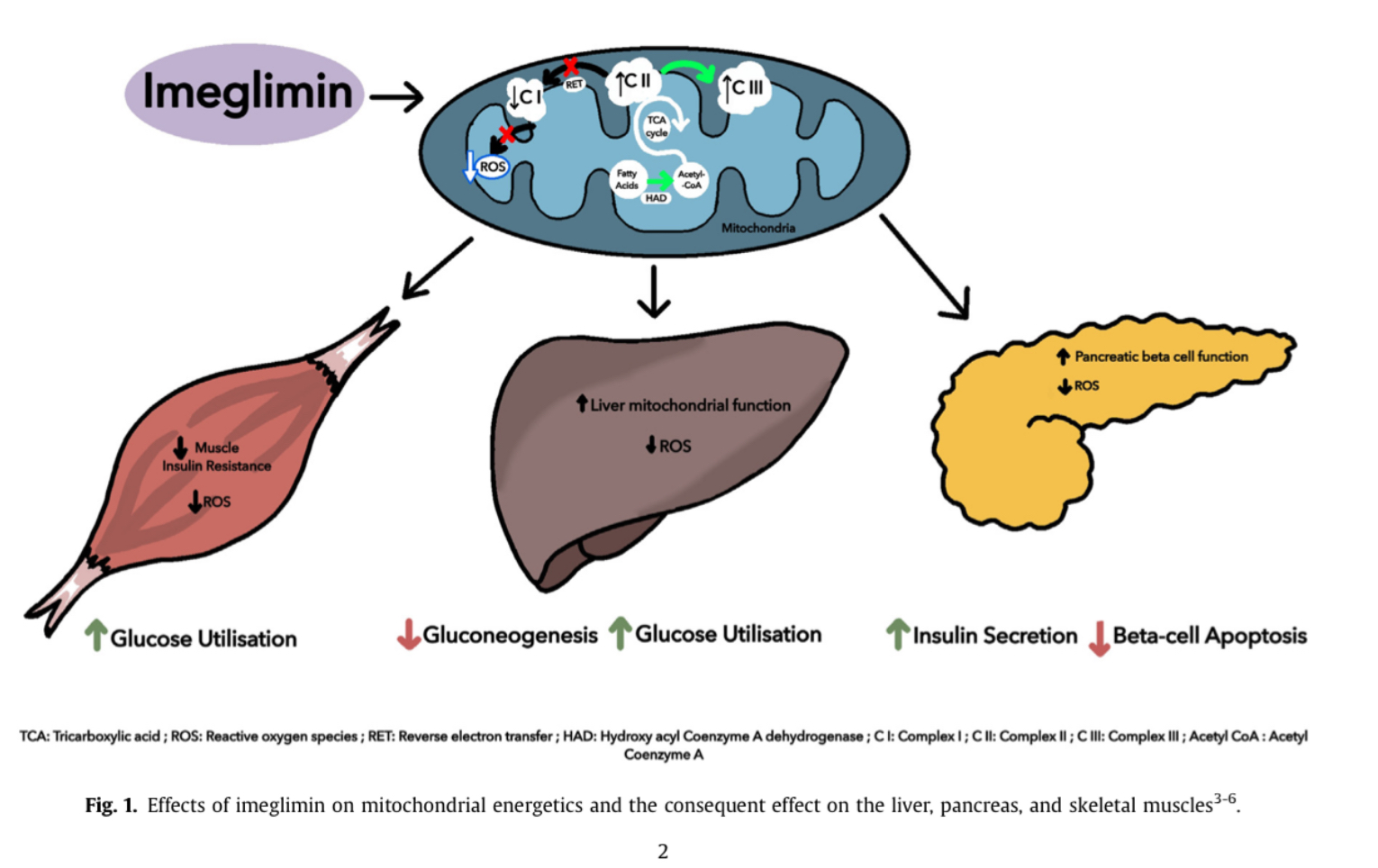
- Q. What is the impact of Imeglimin on Complex I and Complex III on the mitochondrial electron transport chain?
- Imeglimin affects both Complex I and Complex III of the mitochondrial respiratory chain
- In isolated rat hepatocytes, Imeglimin mildly and competitively inhibits Complex I without affecting overall cellular oxygen consumption
- In liver tissue of diet-induced diabetic mice, Imeglimin restores deficient Complex III activity while partially inhibiting Complex I
- It increases the flux through complex I which produces Reactive oxygen species and enhances the flux through complex III
- Q. Tell me more about the Complex I and Complex III of this mitochondrial ETC.
- Complex I and Complex III are two key components of the mitochondrial electron transport chain (ETC), which is also known as the respiratory chain.
- The ETC is a series of protein complexes located in the inner mitochondrial membrane that plays a crucial role in cellular respiration and energy production.
- The ETC consists of four main protein complexes, labeled Complex I, Complex II, Complex III, and Complex IV.
- These complexes work together to transfer electrons from electron donors to electron acceptors, ultimately leading to the production of ATP (adenosine triphosphate), the cell's primary energy currency.
- Q. Is it true that complex I produce more Reactive oxygen species?
- Yes, it is true that Complex I (NADH: ubiquinone oxidoreductase) of the mitochondrial electron transport chain (ETC) is a significant site of reactive oxygen species (ROS) production within the mitochondria. ROS are highly reactive molecules that contain oxygen, such as superoxide (O2•−) and hydrogen peroxide (H2O2). While ROS plays important roles in cell signaling and physiological processes, excessive ROS production can lead to oxidative stress and damage to cellular components, including proteins, lipids, and DNA.
- Complex I is known to produce ROS, particularly superoxide, as a byproduct of electron transfer. Under normal physiological conditions, electrons are transferred from NADH to ubiquinone (CoQ) through Complex I, and protons are pumped across the inner mitochondrial membrane. However, during this process, electrons can sometimes "leak" and react with molecular oxygen (O2) to form superoxide. This superoxide can then be converted to other ROS, such as hydrogen peroxide.
- The production of ROS by Complex I can be influenced by several factors, including the redox state of the electron carriers within the complex, the proton motive force across the inner mitochondrial membrane, and the availability of substrates (e.g., NADH). Additionally, certain pathological conditions, such as ischemia-reperfusion injury, can increase ROS production by Complex I.
- It is important to note that mitochondria have antioxidant defense mechanisms, such as superoxide dismutase (SOD) and glutathione peroxidase, that help neutralize ROS and prevent excessive oxidative stress. However, when ROS production exceeds the capacity of these antioxidant defenses, oxidative stress can occur and contribute to the development of various diseases, including neurodegenerative diseases, cardiovascular diseases, and metabolic disorders.
- Q. What is the impact of Imeglimin on Hepatic gluconeogenesis?
- Imeglimin inhibits gluconeogenesis in isolated rat hepatocytes, similar to metformin.
- Q. What is the difference in terms of the mechanism of action on mitochondria between Metformin and Imeglimin?
- Imeglimin and metformin have divergent effects on Complex I.
- Imeglimin decreases the affinity of NADH for the respiratory chain but does not affect its Vmax (competitive inhibition).
- Metformin decreases both the Vmax and the affinity of NADH for the respiratory chain (uncompetitive inhibition).
- Q. What is the difference between competitive and uncompetitive inhibition?
- Competitive inhibition and uncompetitive inhibition are two different types of enzyme inhibition that affect the activity of enzymes in distinct ways. Here are the key differences between the two:
- Competitive Inhibition:
- Mechanism: Competitive inhibitors bind to the active site of the enzyme, directly competing with the substrate for the same binding site. As a result, they prevent the substrate from binding to the enzyme.
- Effect on Km: Competitive inhibition increases the apparent Km value of the enzyme (Km is the Michaelis constant, which represents the substrate concentration at which the enzyme's reaction rate is half of its maximum rate, Vmax). This is because a higher substrate concentration is required to achieve the same reaction rate in the presence of the inhibitor.
- Effect on Vmax: Competitive inhibition does not change the Vmax value of the enzyme (Vmax is the maximum rate of the enzyme-catalyzed reaction). At high substrate concentrations, the substrate can outcompete the inhibitor for binding to the enzyme, allowing the enzyme to reach its maximum rate.
- Reversibility: Competitive inhibition is usually reversible, meaning that the inhibitor can bind to and dissociate from the enzyme's active site.
- Uncompetitive Inhibition:
- Mechanism: Uncompetitive inhibitors bind to a site on the enzyme that is distinct from the active site (an allosteric site). Importantly, they bind only to the enzyme-substrate complex, not to the free enzyme.
- Effect on Km: Uncompetitive inhibition decreases the apparent Km value of the enzyme. This is because the inhibitor preferentially binds to the enzyme-substrate complex, effectively reducing the concentration of the complex and making it appear as though the enzyme has a higher affinity for the substrate.
- Effect on Vmax: Uncompetitive inhibition decreases the Vmax value of the enzyme. This is because the inhibitor reduces the number of enzyme molecules available to catalyze the reaction, thereby lowering the maximum rate.
- Reversibility: Uncompetitive inhibition can be reversible or irreversible, depending on the specific inhibitor and enzyme involved.
- Overall, the key difference between competitive and uncompetitive inhibition lies in the binding site of the inhibitor (active site vs. allosteric site) and the effects on the enzyme's kinetic parameters (Km and Vmax)
- Q. Does Imeglimin produce the risk of lactic acidosis like Metformin ?
- Imeglimin has mild, competitive inhibition of respiratory chain Complex I
- Imeglimin induces a kinetic constraint on the respiratory chain without affecting the maximal activity
- Metformin is an uncompetitive inhibitor of the respiratory chain and reduces the oxygen consumption rate
- Metformin inhibits mitochondrial glycerophosphate dehydrogenase (mGPD)
- Inhibition of mGPD by metformin leads to inhibition of gluconeogenesis and lactate accumulation
- Imeglimin does not affect rat mGPD compared to metformin
- Metformin, but not Imeglimin, can provoke substantial elevations of plasma lactate
- Imeglimin does not affect increasing cAMP, which is an obligate mediator of incretin action
- Imeglimin modulates mitochondrial function and increases Ca mobilization
- Ca mobilization can be linked to the synthesis and metabolism of NAD
- Available evidence suggests that Imeglimin does not produce the risk of lactic acidosis like Metformin.
- Q. What is the HbA1c reduction seen with Imeglimin?
- Close to 1%
- Q. Does Imeglimin enhance insulin secretion like Sulphonylureas?
- Yes it does
- But the mechanism seems different from Sulphonylurea which is via K ATPase closure
- Q. What is the impact of Imeglimin on cardiac tissue?
- Imeglimin has a potential impact on cardiac dysfunction
- In a Zucker fa/fa rat model of metabolic syndrome, Imeglimin treatment resulted in:
- Decreased left ventricle (LV) end-diastolic pressure
- Increased LV tissue perfusion
- Reduced LV ROS production
- Increased coronary artery endothelium-dependent relaxation
- Q. What is the impact of Imeglimin on kidneys?
- It can reduce interstitial fibrosis and prevent nephron cell death due to Reactive oxygen species
- Q. Does it produce QT prolongation?
- No
- Q. How is it eliminated?
- Via kidney
- Q. What is the standard dose of Imeglimin?
- 1000 mg Twice a day
- Q. What are the dosing recommendations for CKD?
- Indian DCGI suggests:
- GFR <45- not to use the drug
- Japanese regulator
- GFR- 15-45- dose of 500 mg BD
- GFR <15- not to use the drug
- Indian DCGI suggests:
- Q. What is the half-life of Imeglimin?
- 10-20 hours
- Q. How is the oral bioavailability?
- It is fairly good with that of 50-80% and reduces with increasing doses
- Q. Does it have Cytochrome P450-related drug interaction?
- No
- Q. What are the names of the phase III studies of this drug?
- They are the TIMES trials
- Q. Give a summary of the phase 3 trials.
- Phase 3 trials of imeglimin are RCTs evaluating the efficacy and safety of imeglimin 1000 mg BID in people with type 2 diabetes.
- TIMES-1 is a monotherapy trial, TIMES-3 is a combination therapy trial with insulin, and TIMES-2 is an open-label combination therapy trial with other anti-hyperglycemic agents.
- Imeglimin 1000 mg BID is more effective than a placebo in reducing HbA1c levels.
- Q. True or false, when this drug is given with GLP-1 receptor agonist, there is no significant HbA1c reduction?
- True
- Q. Why is there no reduction in HbA1c when the drug is given with GLP-1 receptor agonist?
- This is not exactly known
- It is probably that the patients on GLP1 RA in these trials had failed the use of OAD
- Q. What are the main side effects of Imeglimin?
- Imeglimin has an acceptable tolerability profile based on Phase 2 and Phase 3 studies.
- The most common side effects reported in these studies are nausea, diarrhea, headache, and dizziness.
- No evidence of cytochrome P450 inhibition or induction has been found.
- Imeglimin has no clinically meaningful interaction with food intake based on Phase 1 studies.
- Renal safety studies showed increased exposure to imeglimin due to increased plasma concentration (Cmax) with falling eGFR, especially in CKD Stage 3b and Stage 4.
- No study has been conducted on CKD Stage 5 and patients on hemodialysis.
- Q. What is the longest trial with this drug?
- longest trial has been for 52 weeks
- Q. Can it be given with metformin?
- Yes
- The clinical trials with metformin (especially TIMES 2 ) found additional HBA1c reduction with the drug
- Q. Which are the Imeglimin brands available in India?
- Lupimeg
- Imextor
- Imeglyn
- They are available in doses of 500 mg and 1000 mg
Reference:
- Hallakou‐Bozec S, Vial G, Kergoat M, Fouqueray P, Bolze S, Borel A, Fontaine E, Moller DE. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes, Obesity, and Metabolism. 2020; 23(3):664-673. DOI: 10.1111/dom.14277.
- Singh AK, Singh A, Singh R, Misra A. Efficacy and safety of imeglimin in type 2 diabetes: A systematic review and meta-analysis of randomized placebo-controlled trials. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2023; 17(2):102710. DOI: 10.1016/j.dsx.2023.102710.