- Credits
- Section Writer: Dr. Om J Lakhani
- Section Editor: Dr. Om J Lakhani
Support us:
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
- Support you by Becoming a YouTube member (Click here)
- Q. What are the potential immune-modulatory therapies currently tried in patients with Type 1 Diabetes?
-
- Anti-CD3 monoclonal antibody - Teplizumab in Type 1 Diabetes 1
-
- Low-dose thymocyte globulin
-
- abatacept

-
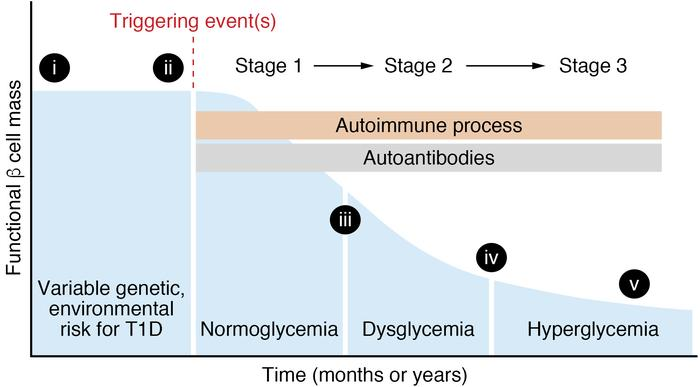
- Q. What are the stages of Type 1 Diabetes?
- Q. What is Stage 1 of Type 1 Diabetes?
- It is when two or more antibodies are positive but the patient is asymptomatic or presymptomatic for Type 1 Diabetes and has normal glucose levels
- The antibodies are :
- GAD65 antibody
- Znt8
- IA-2
- Q. What is Stage 2 of Type 1 Diabetes?
- This is when a patient has
- Antibodies positive (2 or more)
- Glucose and HbA1c are in the Prediabetes range
- Patient is per se presymptomatic or asymptomatic
- This is when a patient has
- Q. What is stage 3 of Type 1 Diabetes?
- Full-blown type 1 diabetes with symptoms
- Q. What is the probability of stage 2 type 1 diabetes developing full-blown type 1 diabetes (Stage 3)?
- 60% in 2 years
- 75% in 4-5 years
- Q. What is Teplizumab in Type 1 Diabetes?
- It is a monoclonal anti-CD3 nonbinding antibody
- Q. What is its role in Type 1 Diabetes?
- It delays the conversion of Stage 2 of type 1 diabetes to stage 2
- Q. Teplizumab in Type 1 Diabetes 1 received approval based on which study?
- Based on study by Harold et al published in 2019[1]
- Q. How was the drug given in this study?
- Daily infusion for 14 days
- Q. How long were the patients followed?
- For 51 months
- Q. How was the progression in the drug versus placebo group?
- The time to diagnosis of type 1 in the placebo group was 24 months
- It was 48 months in the treatment group
- Type 1 developed in 43% of patients on the drug and 72% of patients on a placebo
- Q. What is the potential adverse effect of the drug?
- Lymphopenia
- Q. What was the Protégé study and what were their results?
- This study was published in Lancet in 2011 in which they study the effect of Teplizumab in Type 1 Diabetes 1 in newly diagnosed type 1 diabetes (within 12 weeks of diagnosis)
- Different doses and regimens of the drug were studied
- Overall there was no impact on the primary outcomes in the various groups who were studied - there was no significant beta-cell preservation
- However, a small subgroup did benefit more than others
- They were those who were
- Treated within 6 weeks of diagnosis
- Had C-peptide mean AUC >0.2
- HBA1c <7.5%
- Insulin requirement <0.4 units/kg/day
- Age of 8-17 years
- Hence probably it is more useful if given very early in the course of the disease[2]
- Another study (ABATE study) use the drug in newly diagnosed type 1 diabetes within 8 weeks of diagnosis. Over a longer period, though HBA1c in the two groups was similar, the patients who receive Teplizumab in Type 1 Diabetes 1 had lesser insulin requirement and better beta-cell preservation[3]
- Q. Which is another drug in the same group which is being tried?
- Otelixizumab
- It is showing promising results in some recent trials
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. New England Journal of Medicine. 2019 Aug 15;381(7):603-13. ↩︎
Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011 Aug 6;378(9790):487-97. doi: 10.1016/S0140-6736(11)60931-8. Epub 2011 Jun 28. PMID: 21719095; PMCID: PMC3191495 ↩︎
Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA; AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013 Nov;62(11):3766-74. doi: 10.2337/db13-0345. Epub 2013 Jul 8. PMID: 23835333; PMCID: PMC3806618. ↩︎