- Credits
- Section Writer: Dr. Om J Lakhani
- Section Editor: Dr. Om J Lakhani
Support us:
- Support you by Becoming a YouTube member (Click here).
- Premium Membership- Download PDF version of Notes, Get ad free video and more
- Consultant Membership- Above plus Download Powerpoint presentation of the notes and get access to EndoAI for Free
- Support us by purchasing our book - Click here for more details: Volume 1- THE BEST OF NOTES IN ENDOCRINOLOGY BOOK SERIES
- Other topics for Doctors on "Notes in Endocrinology" - please see ENDOCRINOLOGY NOTES FOR DOCTORS AND HEALTHCARE PROFESSIONALS
- Q. What are the different methods used to prolong the duration of action of insulin in existing basal insulins?
-
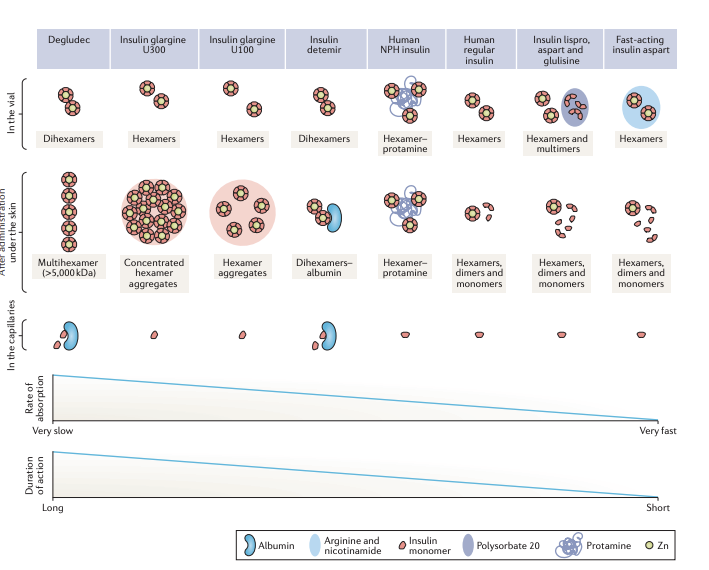
- Degludec
- Present as Dihexamer in the vial and forms a Multihexamer in the skin after administration
- Also has reversible binding to albumin
-
- Insulin glargine
- Presented as a hexamer in the vial and becomes hexamer aggregates under the skin on injection when subjected to physiological pH
- Glargine 300 has more compact and concentrated hexamers further prolonging its action
-
- Insulin detemir
- Dihexamers in the vial
- Binds to albumin reversibly
-
- NPH
- Co-crystalizes with protamine to slow it's absorption
-
- Q. This process of adding lipid chains to insulin, who were the people who first brought this to light?
- Kurtzhals et al
- Q. Can you tell me more about Kurtzhals?
- Peter Kurtzhals and his colleagues played a significant role in the development of insulin detemir (Levemir).
- Kurtzhals and his team were involved in the research and development of this specific insulin analog at Novo Nordisk.
- Their work focused on modifying the insulin molecule to improve its therapeutic properties, particularly its duration of action and stability.
- Q. Which are the two weekly insulin currently under development?
- The two once-weekly insulin formulations currently under development are:
- Basal insulin Fc (insulin efsitora alfa)
- Insulin icodec
- Both of these formulations are in late-phase clinical development
- The two once-weekly insulin formulations currently under development are:
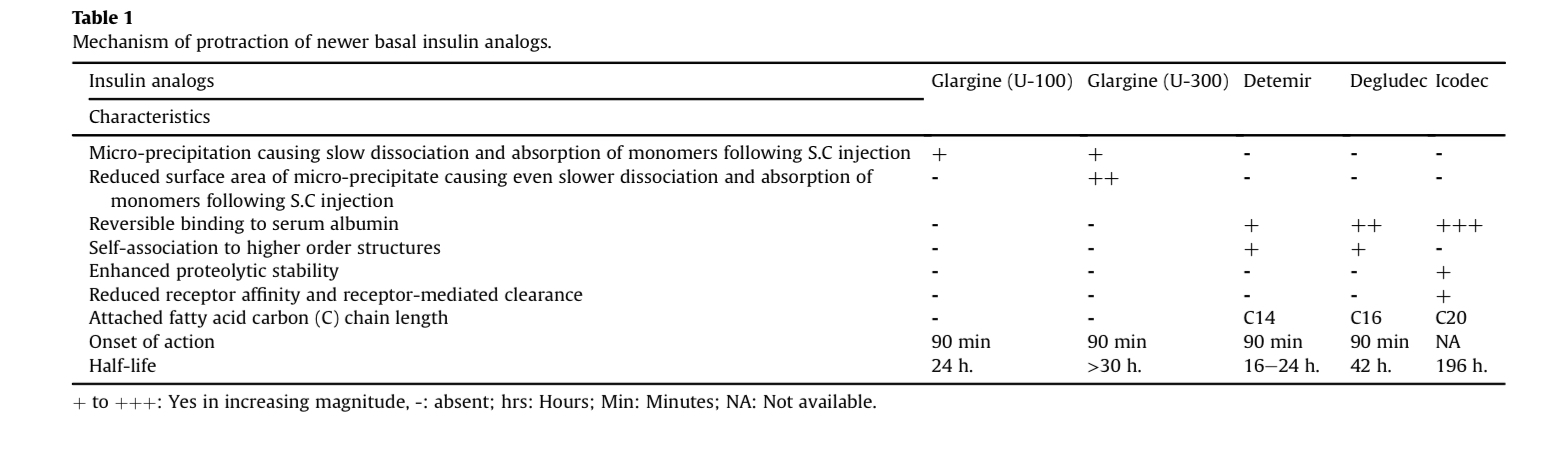
- Q. What is the half-life of Insulin icodec?
- 196 hours
- Q. Tell me more about the pharmacokinetics of Insulin icodec?
- Median time to reach peak plasma concentration was 16 hours after subcutaneous injection
- Mean half-life of 196 hours (More than 8 days)
- Steady-state achieved after 3-4 injections
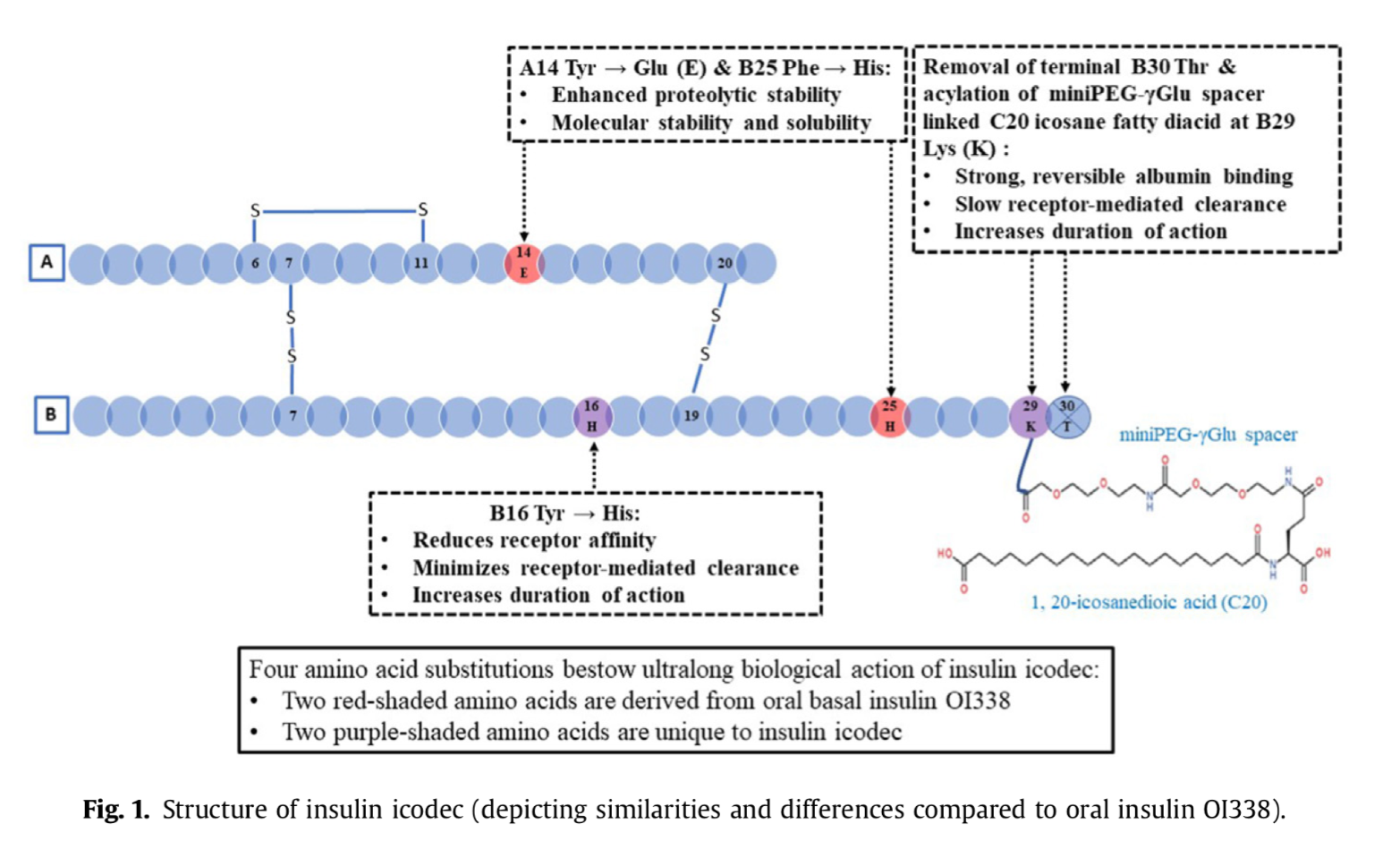
- Q. What is the structural modification done to develop Insulin icodec?
- Insulin icodec has been structurally modified from human insulin by making three substitutions to its amino acid structure.
- Additionally, it has an attached C20 icosane fatty diacid chain.
- This structural modification allows the molecule to bind reversibly to albumin, similar to insulin detemir.
- This modification is crucial as it prolongs the half-life of insulin icodec to 196 hours (approximately 7 days), enabling its once-weekly administration
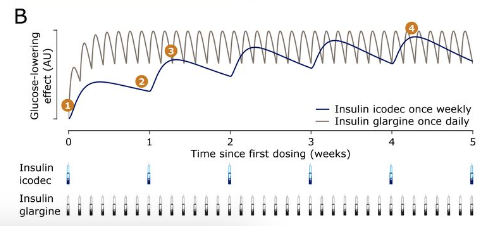
- Q. Can you depict with a diagram how the insulin AUC builds up in Icodec vs Glargine
- Q. Can you explain in simple words for a layperson's benefit how the insulin icodec has a prolonged half-life and duration of action?
- Albumin Binding: Insulin icodec is designed to bind strongly to a protein in the blood called albumin. Albumin is abundant in the bloodstream and acts like a carrier or a 'taxi' for various substances. By hitching a ride on albumin, insulin icodec stays in the bloodstream for a longer time instead of being quickly broken down or removed.
- Stable Structure: The molecular structure of insulin icodec has been tweaked to make it more stable. This stability prevents it from being rapidly degraded or eliminated by the body's natural processes that normally break down and clear insulin.
- Controlled Release: These modifications also allow for a more controlled and gradual release of insulin into the bloodstream. This means that instead of a quick spike and drop in insulin levels, insulin icodec provides a steady, prolonged effect.
- Q. What is the difference in terms of which carbon was used to attach the fatty acid side chain?
- Detemir- C-14
- Degludec- C16
- Icodec- C20
- Q. Since insulin icodec binds with albumin, will hypoalbuminemia lead to clinical issues?
- No
- this is because each albumin has at alteast 4 high-affinity binding sites
- Additionally, the serum concentration of insulin is far lower than the number of available albumin sites - there are 2000 fold more albumin binding sites
- Q. Is it true that insulin icodec has less affinity for insulin receptors?
- Yes this is true
- Because of the less affinity- it's clearance is also slow
- However, the trick is to maintain potency with less affinity to the receptor
- This is quite counterintuitive to what is traditionally thought of in pharmacology- however it solves the purpose
- Q. What amino acid substitution in Insulin icodec solves the problem of binding to the insulin receptor?
- The substitution of native tyrosine at B16 to His
- This reduces the receptor affinity
- Q. Again, is this true that a lot of the amino acid substitution ideas for insulin icodec come from the oral insulin project?
- This is indeed true
- Some of the concepts for insulin icodec comes from Novo Nordisk's oral insulin project OI388
- A14 Tyr → Glu and B25 Phe → His both come from Oral insulin project
- Q. If were developing oral insulin, why was the project not taken forward?
- The OI288 showed good fasting glucose similar to once-a-day insulin glargine
- However, to achieve equivalent doses as insulin glargine, the amount of insulin required by OI388 was too high and hence the entire system was not commercially viable
- Q. Why are amino acid changes mainly made in the beta chain of insulin and not the alpha chain?
- This is because of the risk of oncogenic potential
- Alpha chain substitution especially in the first 10 amino acids leads to an increase in oncogenic potential
- This is why, changes are reserved to beta-chain
- In this case, change was made in the alpha chain but at A14 and not in the first 10 amino acids
- Q. It reaches a steady state after how many injections?
- It achieves a steady state after 3-4 injections
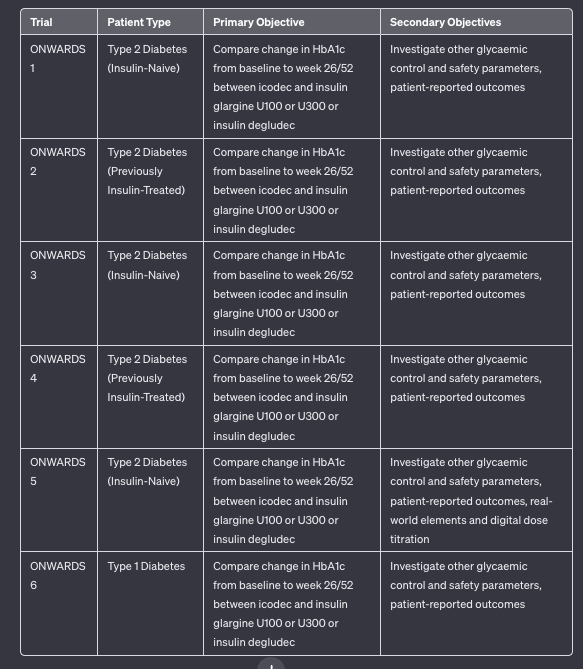
- Q. What are the names of the clinical trial series for Insulin icodec?
- the ONWARDS trial series
- Q. Enlist the various ONWARDS trials for Insulin icodec
- Q. True or false, one unit of insulin icodec is as effective as 1 unit of basal insulin given daily?
- True
- It is unit-to-unit equal in potency
- Hence the dosing of the insulin to be given is 7 times the dosing of the usual basal insulin
- Q. What is the concentration of Insulin icodec?
- The concentration of insulin icodec is 700 U/mL. This is seven times the concentration of a typical 100 U/mL daily basal insulin.
- The higher concentration is necessary to maintain the equivalent potency in a once-weekly dose compared to daily basal insulins, with the same injection volume
- Q. In terms of Hba1c reduction, is insulin icodec superior, the same, or inferior to other basal insulins?
- Insulin icodec has shown superiority in terms of HbA1c reduction compared to other basal insulins in certain patient groups. Specifically, it demonstrated superior HbA1c reduction in insulin-naïve patients (in the ONWARDS 1, 3, and 5 trials) and in patients switching from daily basal insulin in the ONWARDS 2 trial.
- In the ONWARDS 4 trial, insulin icodec was found to be non-inferior to insulin glargine U100 in patients on basal-bolus insulin, indicating similar efficacy in terms of HbA1c reduction
- Q. What about the risk of hypoglycemia?
- In the ONWARDS 1-5 trials involving patients with type 2 diabetes, insulin icodec showed similar rates of combined level 2 and level 3 hypoglycemia compared to the control groups using daily basal insulins.
- However, in the ONWARDS 6 trial with patients having type 1 diabetes, the rate of hypoglycemia was higher for icodec compared to daily degludec at 26 weeks, despite similar glycaemic control. This suggests an increased risk of hypoglycemia in type 1 diabetes patients when using insulin icodec.
- Clinical concerns remain regarding the potential for recurrent hypoglycemia with insulin icodec, given its long duration of action and the limitation of down-titrating the dose only once weekly.
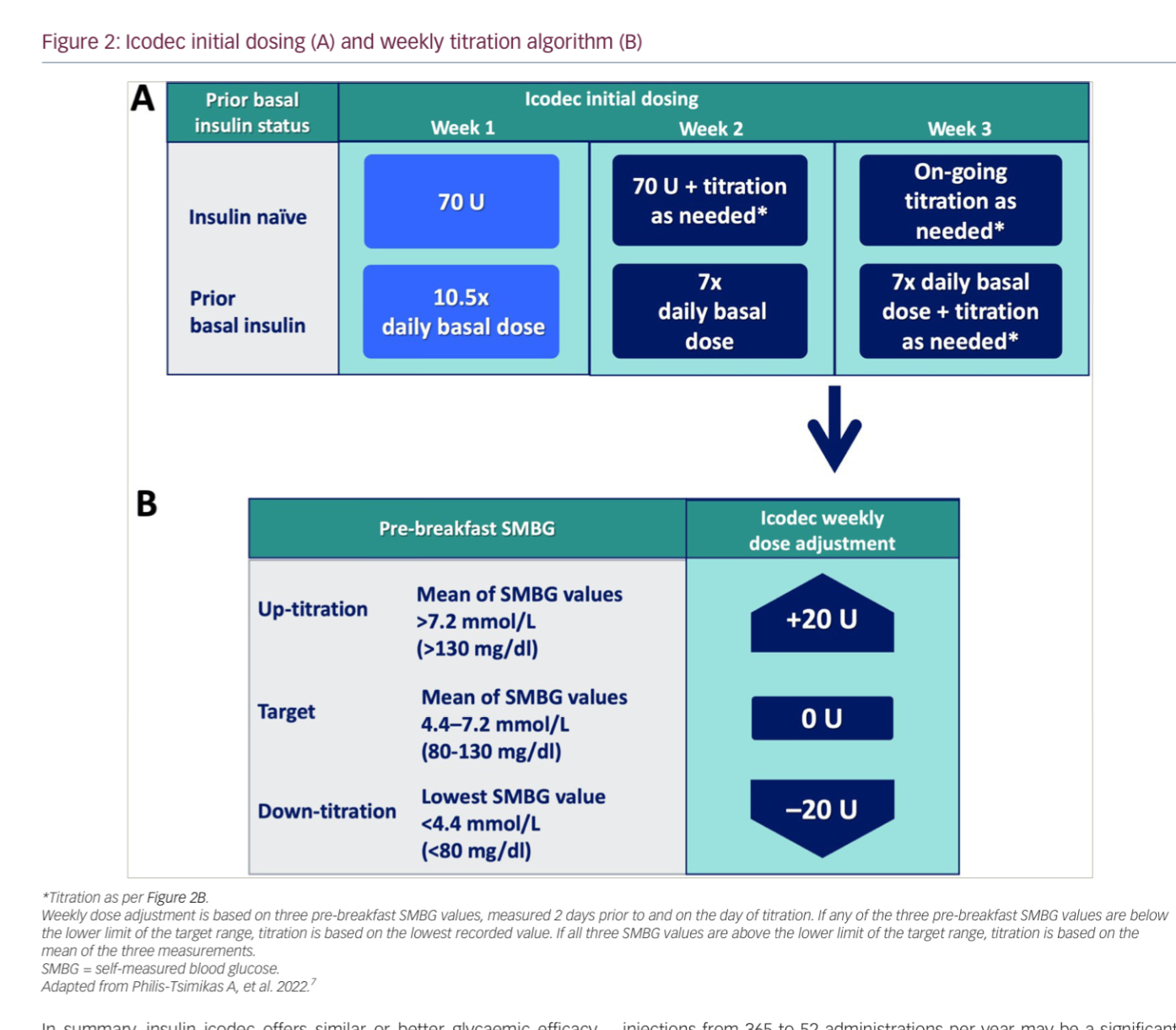
- Q. What is the suggested starting dose of Insulin icodec in insulin-naive patients?
- 70 units/week
- This is 7 times the usual basal insulin starting dose which is 10 units
- Q. What is the starting dose when shifting from a daily to a weekly dose?
- You need to give 10.5 times the current basal insulin dose as the starting dose
- this is 7 times the current dose plus an additional 50% (3.5 times) as a loading dose
- Q. How do you titrate the dose?
- Titrate by 20 U weekly if fasting glucose is above or below the target in the 3 days before the next injection
- Q. Summarize the starting and titration of Insulin icodec.
- For Insulin-Naïve Individuals with Type 2 Diabetes:
- Start with a dose of 70 U weekly, which is equivalent to 10 U of daily basal insulin.
- The injection volume is the same as 100 U/mL daily basal insulin due to the 7× higher concentration (700 U/mL).
- Titrate by 20 U weekly if fasting glucose is above or below the target in the 3 days before the next injection.
- When Switching from Established Basal Insulin:
- The initial dose is 7× the previous daily dose of basal insulin.
- Add a one-time additional 50% of the calculated once-weekly dose (10.5× daily basal dose) for the first week.
- From week 2, the recommended dose is 7× the previous daily dose.
- Continue with ongoing weekly titration by 20 U adjustments from week 3 onwards.
- For Insulin-Naïve Individuals with Type 2 Diabetes:
- Q. What happens if the patient misses the dose on the requisite day of the week when she takes it?
- The patient has a 4-day window to take the missed dose
- However, the next dose is to be taken as per schedule
- Q. Is dose adjustment required in hepatic or renal impairment?
- No
- Q. What is Icosema?
- Combination of weekly insulin icodec with weekly semaglutide
- Recently started phase I trials
- Q. What are the names of the clinical trials for icosema?
- COMBINE trials
- Q. Overall what are the pros of using Insulin icodec?
-
- Reduces the number of needle pricks from 365 to 52
-
- Better HbA1c reduction and time in range in Type 2 Diabetes based on the ONWARDS 1-3 trials without risk of hypoglycemia
-
- Potential weekly combination with Semaglutide - cinema
-
- Improved compliance
-
- Q. What are the cautions with Insulin icodec?
-
- Higher hypoglycemia in type 1 in the ONWARD 6 trial
-
- Protocol for what is to be done in case recurrent hypoglycemia develops for patients on insulin icodec is not known.
-
- Longer time to achieve steady state - hence little use in severe hyperglycemia with glucotoxicity
-
- Q. Tell me more about the Basal insulin Fc (BIF) the other weekly basal insulin under development
- Composition: Basal Insulin Fc (BIF, insulin efsitora alfa, LY3209590) is a fusion protein of a single-chain insulin variant and a human IgG Fc domain.
- Design Goal: Developed for once-weekly administration, offering a more convenient option than daily insulin injections.
- Pharmacokinetics: Features a low weekly peak-to-trough ratio with a half-life of 17 days, leading to more stable glucose levels.
- Clinical Testing: Undergoing clinical testing for both type 1 and type 2 diabetes mellitus.
- Phase 2 Study Results: Showed non-inferior glycemic control compared to daily insulin degludec in type 1 diabetes patients, without increasing hypoglycemia risks.
- Safety Profile: Comparable to daily insulin degludec, with similar rates of hypoglycemia and serious adverse events.
References:
- Bajaj HS, Goldenberg RM. Insulin Icodec Weekly: A Basal Insulin Analogue for Type 2 Diabetes. touchREVIEWS in Endocrinology. 2023 May;19(1):4.
- Mathieu, C., Gillard, P. & Benhalima, K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol 13, 385–399 (2017). https://doi.org/10.1038/nrendo.2017.39
- Singh AK, Singh A, Singh R, Misra A. Once-weekly basal insulin icodec: Looking ONWARDS from pharmacology to clinical trials. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2022 Sep 1;16(9):102615.